Microfluidics in industry
In this article of our series on microfluidic lab-on-chips we discuss some basic principles of how to detect or quantify the amount of biochemical target in a test sample.
The detection principles consist of one or more of the following elements:
- the bioreceptor that can bind very specifically to the target,
- a way to increase the concentration of the target, to make the test more sensitive,
- an optional marker molecule connected to the bioreceptor to facilitate detection,
- a sensor that can measure the presence or even the amount of the bound target.
Bioreceptor
Developing a suitable bioreceptor belongs to the field of biochemistry. It is important that the bioreceptor is selective, only bonding to the target and not to similar molecules in the sample, causing false positive results. The bioreceptor usually is an enzyme, an antibody or a nucleic acid.
Concentration of the target
Next important aspect are the concentration techniques, in order to increase the signal to be measured and thus making detection more reliable.
One way to increase the concentration is to “amplify” the target in the sample. This is possible when the target is a nucleic acid, such as DNA or RNA. With the polymerase chain reaction (PCR) the specific part of the DNA/RNA that we need to detect can be replicated, doubling the amount of target in the sample. By repeating this e.g. 30 times, the amount of target is multiplied 1 billion times!
Another way to increase the concentration of target is to apply the bioreceptor on a specific detection surface. This surface can be a fixed surface in the detection zone of the microfluidic chip. The advantage of the microfluidic environment is that there is a large surface to volume ratio, making it much more likely for the target molecules to come in contact with the bioreceptors on the surface than in a macroscopic test environment.
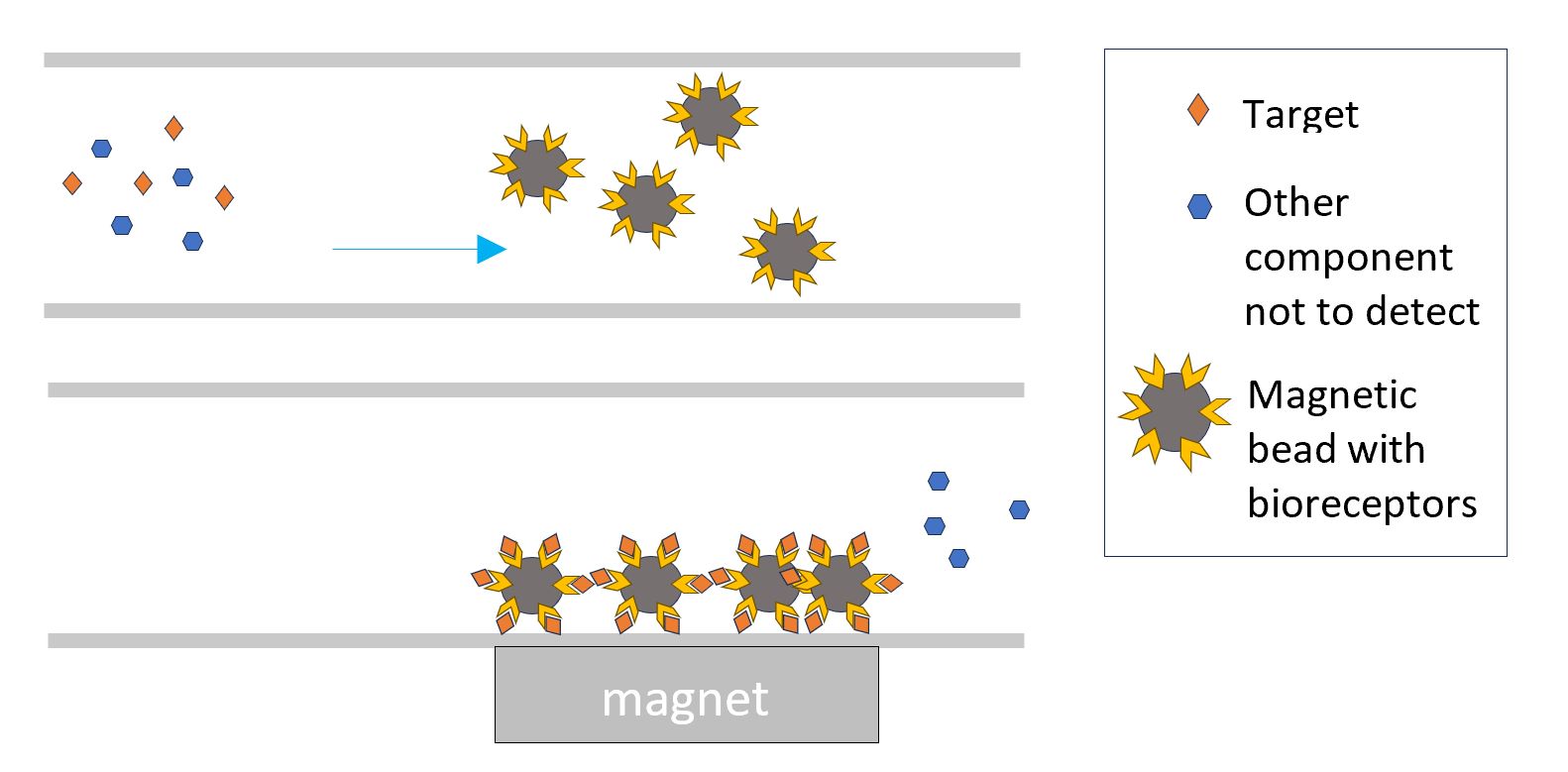
The bioreceptor can also be bound to magnetic particles. The interaction of the target with the magnetic particles can happen over the entire volume instead of only at a fixed surface. By attracting in a next step the magnetic particles to a detection zone in the chip, the detected target is concentrated in that zone.
Similarly the bioreceptors can be bound to large particles that can be caught in a filter element and hence concentrate the target.
Detection of the target
After concentrating the bound target, the next step is to detect it. Here too, there are different options.
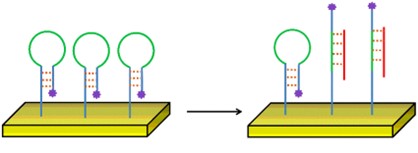
The bioreceptor can have a fluorophore bound to it, in a way that it is only visible when the target is bound to the bioreceptor (e.g., a hairpin probe). This is called a labelled detection.
Or the bound target or bioreceptor has electrochemical properties, which can be measured by amperometry, impedance measurement, potentiometry or voltammetry.
The electrical properties of the detection surface (resistance, capacitance, inductance) can change according to the amount of bound target.
Mechanically, the detection surface can deform by the extra weight of the bound target or its resonance frequency can shift.
The target itself or the activated marker on the bioreceptor can fluoresce or emit light. It can change the optical properties of the detection surface by absorbing, scattering or refracting incident light. In the most simple case, the detection surface changes colour when more bound target is present. Other techniques also enable very detailed analyses by measuring variations in the refractive index of a surface as a function of the species that bind to it (e.g. surface plasmon resonance).
Sensor
The final step is then to use a sensor to detect/monitor/quantify this fluorescence, light intensity or spectrum, or the changing electrical, mechanical or optical property of the detection surface.
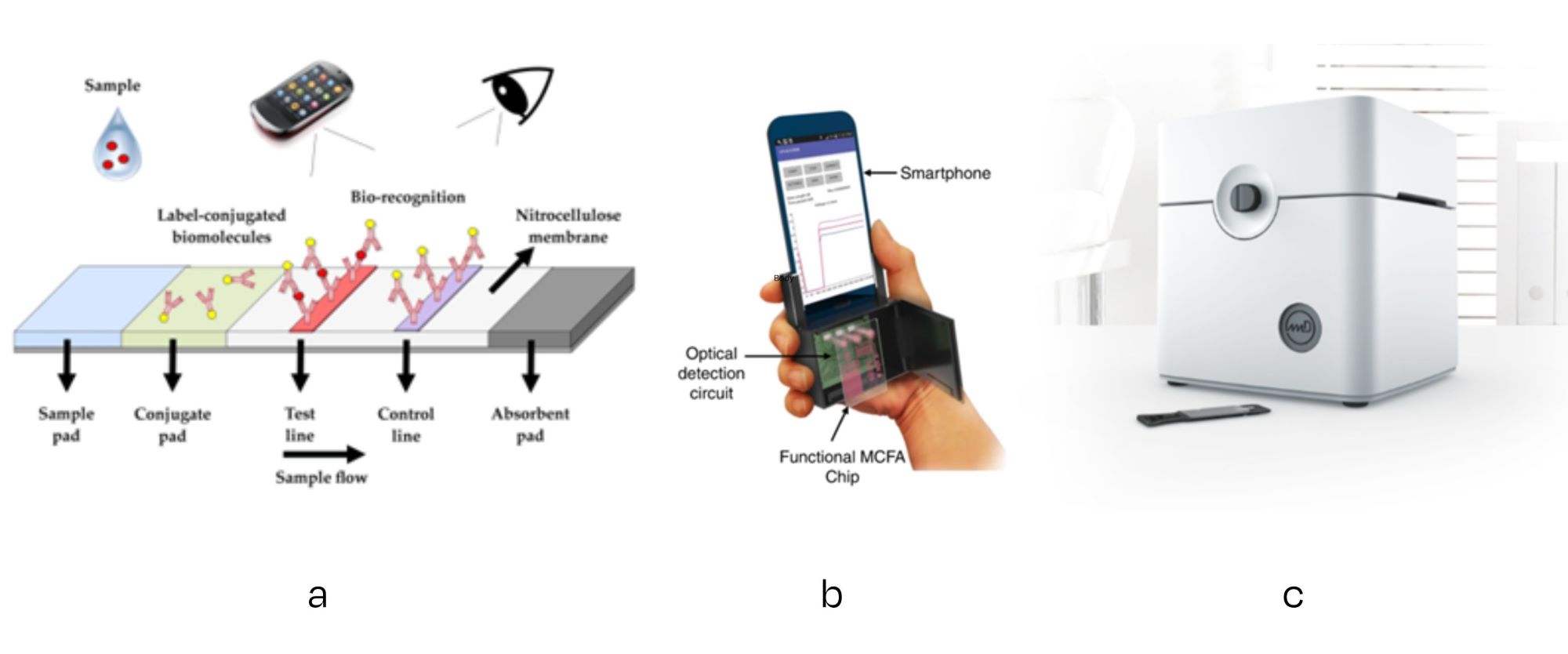
In the most simple solution of colour change detection, the user of the test can see with his/her bare eye the result of the test (like a pregnancy test, covid self test or swab tests for hygiene monitoring). A next level could be the use of a smartphone to interpret optical changes in the detection zone more quantitatively.
More advanced solutions will consist of custom-built actuator/sensor combinations, in order to apply the required excitation (voltage/current/vibration/light) and measure the response (electric, electrochemical, mechanical, optical). Some solutions can be realised relatively simple and at a low cost, e.g., a lighting-optics-camera for optical sensing.
In a nutshell this is how biological targets are detected in a sample, irrespective of the type of application (medical diagnostics, plant, animal, environmental, food tests, …) and how this detection process can be integrated in a lab-on-a-chip.
In previous articles we have already discussed the design steps and the prototyping techniques for the development of the dedicated microfluidic chip. We also presented a camera based solution to control the flow in the chip. In the next article we will zoom in on the aspect of thermal control in the chip, a critical factor in performing PCR.
In future articles we will zoom in on aspects of the flow control and thermal control in the chip.
Do you have a lab-on-a-chip idea? Knowledge and production facilities to develop and produce these products locally, are available in Belgium. We can help you to get started with your idea.
Project funding
VLAIO COOCK Medical diagnostics goes micro and smart, HBC.2021.0560
More information about the project
Medical diagnostics goes micro and smart
Would you like to know more?Or maybe you already have a lab-on-a-chip idea? We can help you to get started! Today, knowledge and production facilities are available in Belgium to develop and produce lab-on-a-chip systems locally. |