Medical diagnostics goes micro and smart | Micro and smart automation technologies for the development of point-of-care diagnostic and testing devices
The 'Medical diagnostics goes micro and smart' project accelerates the availability of micromanufacturing, smart automation technology and development methods to Flemish companies for the development of lab-on-a-chip systems for point-of-care diagnostics.
Context
Lab-on-a-chip solutions and smart test equipment for point-of-care diagnostics
Quality healthcare services require the development of high-performance diagnostics and testing equipment for the detection of specific viruses, proteins, cells, nucleic acids, metabolites, etc., in the context of a variety of pathologies. In this area, speed prevails if we are to better manage patients' health and tailor therapies more precisely to need. For in vitro diagnostic tests (IVD) performed in centralised laboratories, patients usually have to wait several days to get their test results.
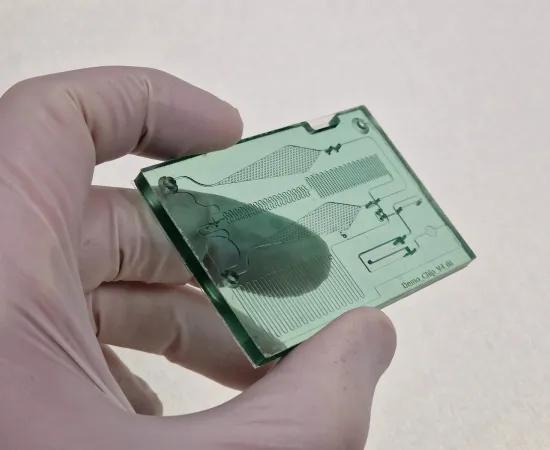
Several micromanufacturing and smart automation technologies can be used in industrial settings today to develop lab-on-a-chip solutions. Tailored to a specific application, lab-on-a-chip systems combine a low-cost, single-use polymeric microfluidic chip with a portable reader. These bring accurate, low-cost diagnosis to the patient (point-of-care), use only a fraction of sample volume and reagents (µl) and deliver accurate test results quickly.
Huge economic potential for point-of-care diagnostic solutions for various pathologies:
Medical diagnostic tests will continue to play a main role in healthcare in the future. Studies have shown that the Benelux region may well become a leading life sciences hub and have outlined the great potential of microfluidics in various applications. The crossover collaboration between biotechnology and emerging digital, micro- and nanomanufacturing technologies offers Belgium an annual growth potential in the number of direct life sciences jobs of 3.4 per cent, reaching 50,000 FTE by 2030. This growth market is still in an early adoption phase and requires strengthening the capacity of Flemish companies to accelerate such innovations.
The development of a lab-on-chip system requires efficient collaboration between several expert fields in new value chains that are yet to be formed. The technological challenges require, on the one hand, a knowledge leap for the start-up with recent technologies and, on the other hand, close cooperation between biotech companies and technology partners for the engineering, development and production of both the microfluidic chip and the readout device.
Objective and results
The 'Medical diagnostics goes micro and smart' project accelerates the availability of micromanufacturing, smart automation technology and development methods to Flemish companies for the development of lab-on-a-chip systems for point-of-care diagnostics.
Conversion research demonstrates the potential of SME deployable technology. The result will be pragmatic methodologies, tools and guidelines for SMEs to support companies in the development process of a microfluidic chip and the portable readout device, the required close interaction between the two developments and the specific challenges in starting up with this technology. Results include:
- An overview on the deployable state of technology for micromanufacturing and smart automation.
- A systematic approach to developing lab-on-a-chip solutions in a multidisciplinary team.
- Methodologies, knowledge and guidelines for the specific development aspects: microprocessor design, microfluidic chip design, design and production of moulds with microfeatures, microinjection moulding of products with microfeatures, mechatronic design of the readout device, verification techniques for the chip and the readout device, estimation of technological and economic feasibility.
- A demonstrator based on a use-case for the development of lab-on-a-chip solution with a low-cost microfluidic chip made of a biocompatible single-use plastic and an associated portable automated readout device. This demonstrator goes through the entire development process (up to prototype and verification) and also illustrates the interaction between the stakeholders (biotech companies and their engineering and manufacturing partners). Proofs-of-concept demonstrate the potential of technologies which can be used by SMEs.
Knowledge transfer makes the target group aware of the innovation potential, ensures in-depth knowledge transfer and brings together all stakeholders involved in this value chain.
Approach
The project provides an answer to both aspects that are important for accelerating technology uptake: knowledge transfer across disciplines and bringing complementary companies together. This allows each company to focus on (incremental) innovation from its core competency and rely on partners for the other competencies.
Target group
Sirris and MEDVIA aim to bring together and support all those involved in this value chain of biomedical, diagnostic applications: biotech and pharma companies (spin-offs, scale-ups and established companies, mainly SMEs) and technology companies that can develop and manufacture the physical medical solutions. This includes, on the one hand, the design and production of the chip (micro-injection moulding, micromachining and mould making, assembly) and, on the other hand, the engineering and production of the readout device.
Reference
VLAIO COOCK Medical diagnostics goes micro and smart, HBC.2021.0560



