New IVDR regulation explained for developers of in-vitro diagnostic medical devices
Within the context of the next supervisory group meeting of the COOCK project 'Medical diagnostics goes micro and smart' a seminar will be organised by one of the member companies. This part of the meeting will therefore be open to third parties and companies not involved in the project.
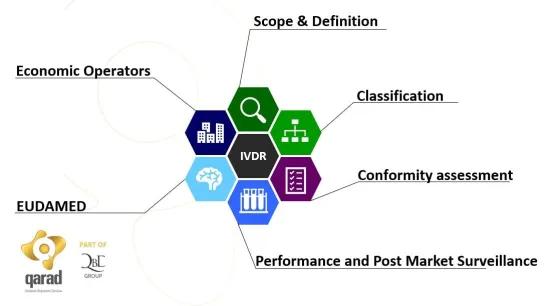
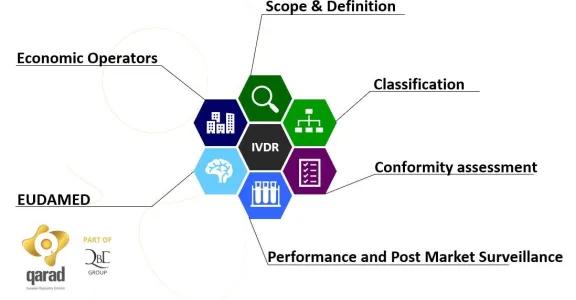
During this seminar we will focus on the application of the Regulation on microfluidic chips and on their conditioning and read-out devices meant for diagnostic testing.
Further information on the speaker (Qarad) and the IVDR regulation can be found on our blog
For whom?
The seminar is aimed at companies manufacturing the systems and accessories for such in-vitro diagnostic medical devices, including their suppliers and service providers.
Participants fee
The seminar is free for members of the supervisory group of the COOCK project 'Medical diagnostics goes micro and smart'. Other participants pay 200 euro (excl. VAT).